Relating Some High Points in Photocell Progress |
||
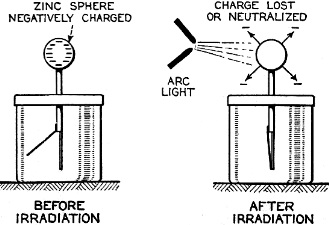
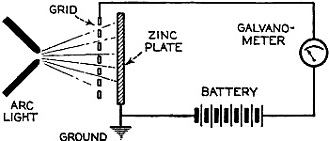
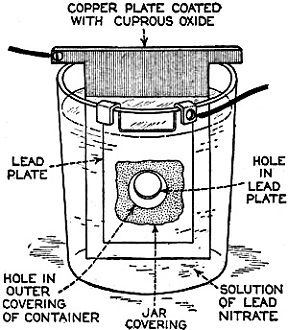
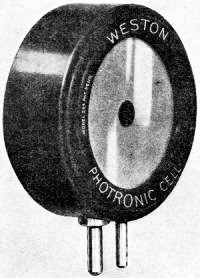
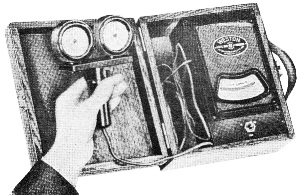
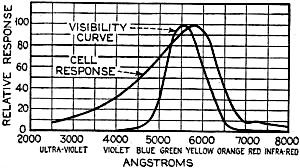
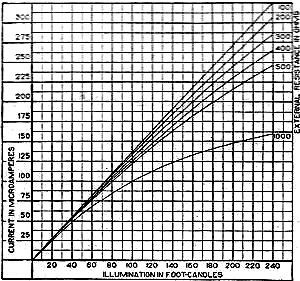
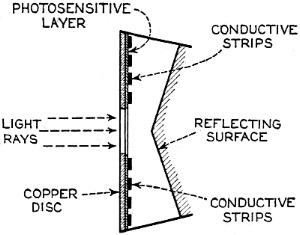
Edward Weston was a pioneer in the photoelectric cell field. His "photronic" cell was one of the first successful devices for commercial use. Just like with early battery cells, photoelectric cells of the era required a liquid medium to facilitate electron transfer and thereby generate electric current. The lead nitrate compound used by Weston is now considered a possible human carcinogen. Mr. Brooke Clark has a web page with extensive data on the history of Weston's photoelectric sensors, meters, test data, patents, and history of his company - which now has the name Huygen Corporation. Photoelectric science has advanced significantly in the 80 years since this article was published. A good website to visit regularly if you like following progress on photocell technology is Semiconductor Today. Relating Some High Points in Photocell Progress Figure 1 - The photo effect of light on a charged sphere connected to an electrometer. At the left is the sphere before radiation, showing the gold-leaf repel, and at the right a sphere acted upon by light from an arc with the gold-leaf at rest alongside the electrode. More and more experimenters and technicians are turning their efforts toward experimentation with light-sensitive apparatus for the control of machinery for detecting bodies, for counting and sorting manufactured products and for work in color matching, etc. The author in this article points out some of the fundamental principles upon which photo-electric devices operate. He also devised them into classes and relates some of the outstanding developmental achievements to date. By Irving J. Saxl, Ph.D. The wide technical application of light-sensitive devices in the fields of radio, television, sound film, for controlling industrial processes and chemical actions; finally the creation of electric energy directly from light have brought about the development of many forms of photo-sensitive apparatus. When Heinrich Hertz, as early as 1887, discovered that the length of electrical discharges as produced by an electrical inductor could be increased when the electrodes between which the spark passed were illuminated by the light of a mercury interrupter, he probably did not dream of the wide industrial and scientific possibilities opened by his discovery and the modern light-sensitive cells which are several hundred times more efficient than the crude device in which he recognized the physical fundamentals of the photo-electric effect.Early Photo-Electric Effects Figure 2 - Stoletow's experiment, showing that a zinc plate will give off electric particles when illuminated, the particles passing from the zinc plate to the grid and measured by a galvanometer. Photo-Electric Effect What does this wonder tool of the electronic magician of today consist of? The theory of all types of light-sensitive devices - and there are today quite a number of widely different types - goes back to the common source: the photo-electric effect. Under the influence of electromagnetic radiation, preferably of a frequency which can be seen with the human eye, certain reactions take place in the irradiated material. This law, however, is more general than just for the range of human visibility. Light and other electromagnetic waves beyond the range of light have an effect upon almost everything in the universe. Light colors the leaves of the trees green by developing chlorophyll particles in them; it influences the halogenides of silver in the emulsions of our photographic films; it sets up a voltage difference in biological material which is irradiated; it acts, with a mechanical force of attraction or repulsion, upon the tails of comets being driven away by the sun's radiation. Mechanical and chemical reactions are inaugurated under the electromagnetic radiations of certain wavebands, a part of which are of this dimension we humans call color. What physical data do we have about these reactions? It was William Hallwachs1 who took the essential parts of Hertz's arrangements and simplified his experimental set-up so far that he was able, in 1888, to clarify the problem of photo-electric reaction. This was the thought of Hallwachs: if the light of the mercury interrupter, shining upon the electrodes could seemingly effect the distance between the electrodes, then probably something was going out from the electrodes which reduced the resistance of the space between them. From were could this something originate, if not from the electrodes themselves? Electrodes, at that time, were made of zinc. So for isolating this phenomenon, Hallwachs put a polished zinc sphere upon a sensitive gold-leaf electroscope which he charges negatively. The leaves, ordinarily showing a wide divergence, under these circumstances touched each other almost instantly when the electroscope was irradiated with an arc-light. The negative charge had disappeared, or a positive charge had been acquired, thus neutralizing the system. See Figure 1. In further investigations, using a sensitive quadrant-electrometer instead of an electroscope, he and other investigators - especially Righi and Stoletow - were able to show that under the influence of electromagnetic waves physically similar to radio waves but of a considerably higher frequency, a negatively charged body loses its charge, whereby such charge outgoing from the bod follows practically the lines of electrostatic force! Early Experiments In Stoletow's experiment a polished zinc plate Z (see Figure 2) was exposed to an intensive light source which included the shorter wavelengths. Outgoing negative charges were collected upon a grid G, thus diminishing the space resistance between Z and G and allowing a small current to pass through the sensitive galvanometer G under the influence of the electromotive force delivered by the battery. This circuit of Stoletow2 actually disclosed the principles used in many modern photo-electric circuits. However, the energy output of his polished zinc plate, with relation to the light falling upon it, was a small one and inconstant. For increasing the photo-electric sensitivity, several methods were later followed. First, it was realized that only a polished plate acted satisfactorily. As soon as this plate dulled, mainly by covering itself with a layer of zinc oxide, the photo-electric effect ceased. Figure 4 - The new Weston photronic cell. Sensitive Relay Figure 5 - A contact galvanometer of a type which operates directly from a disc cell. Elster and Geitel3 used, instead of the pure zinc, an amalgamation of zinc and alkaline compounds. Their sodium amalgam, which was enclosed in a glass vessel to prevent oxidation, may be considered as the first photo-cell in use, although it tarnished badly and had to be cleaned by a magnetic scraper. Finally, by discovering that colloidal suspension of the cathode increased the sensitivity by two decimal points, they were able to build a cell which, in many ways, has not been changed considerably in its fundamentals until recently. The increase of sensitivity was produced mainly by passing a glow discharge in a photo-cell filled with hydrogen gas, to evacuate the cell after the sensibilization and to fill it with an inert gas as argon or helium of low pressure.4 There are today widely different types of light-sensitive devices which, though they go back to the same fundamental principles, are widely different in their action and appearance. From the various developments along the lines of light-sensitive cells, the following are of practical interest: 1. Photo-electronic Cells. These are the cells most widely used. although for many purposes other types of cells seem to be promising. 2. Wet Photo-voltaic Cells, using the Becquerel effect, who discovered (in 1839) that a potential difference is created between two electrodes in an electrolyte from which one was irradiated and the other kept in the dark for a period of time. 3. Dry Photo-voltaic Cells ("Light batteries," disc cells with copper oxide, silver selenide. etc.) 4. Photo-conductive Cells (Selenium bridges. for instance). These have been neglected for some time, but their improved types are now most promising. 5. Crystal Photo-cells (effects between minerals, as. for instance, argentite, and their metal contacts). In this article special attention is given to the newer types of dry and wet photo-voltaic cells. How to Make Photo-Voltaic Cells Photo-voltaic cells are found in nature. Every leaf, if irradiated, indicates minute voltage changes. These, however, are too low to be measured without special amplification. But certain chemical compounds have been developed with which it is possible to make a photo-voltaic cell (Becquerel cell) for a few cents. Figure 3 shows a diagrammatic sketch of such a light-battery. A beaker of clear glass is filled with a solution of lead nitrate. In this lead solution is immersed a copper plate which has been uniformly coated with cuprous oxide. At the front of the beaker a lead plate is hung into the solution. The lead plate has a hole in the middle through which the light can enter the cell. The outside of the cell is covered with black paper or coated with a dark lacquer with the exception of a hole at the same place where the hole in the lead plate is. The cell is protected at all sides from light with the exception of that one opening. If the copper disc is irradiated through this hole, a voltage difference is produced between the lead and the copper electrode. If the two poles are connected in an electric circuit, currents as high as several milliamperes can be produced when the cell is exposed to daylight or sufficient artificial illumination is brought into the cell, for instance, by concentrating the light of an incandescent lamp by a lens before the hole. The amount of current output for a given intensity of light depends to a large extent upon the electrode surface, larger surfaces giving stronger currents. Besides cuprous oxide, it is possible to use silver halides and many more substances. Even two plates of the same material, one of which is exposed to the light and the other kept in the dark, show the photo-voltaic effect, although not quite so intensively. Investigations made by Garrison5 indicated that the current produced between the electrodes reverses its direction shortly after the illumination of one plate and flows thereafter in the opposite direction, finally reaching equilibrium. A Disc Cell Illuminometer Figure 6 - View of a commercial illuminometer that operates without batteries. The device uses two disc cells and is calibrated directly in foot candles Frequency Response Curve Figure 7 - Response curve of the photronic cell using a quartz window up to 4000 Angstrom units and a glass window for the longer wavelengths Current Output Curves Figure 8 - This chart shows the current output curves of the photronic type of photo-electric cell and shows its interdependence with external resistance. The curve is calibrated in illumination foot candles and current in microamperes. The Disc Cell Figure 10 - Schematic cross-section view of the disc cell illuminated through a circular opening at the back. Audio-Frequency Response Figure 11 - Curve showing audio-frequency response of a disc cell operating a light source interrupted at different audio frequencies. It is also possible to produce this voltage difference without the aid of an electrolyte. With he aid of discs covered with thin layers of material it is possible (similar to the wet photo-voltaic cells based upon the Becquerel effect) to develop currents directly from sunlight. Considerable research has been done on this idea in the last year. Dr. Lange of Berlin experimented with copper-oxide discs and increased the output of his cells considerably by using silver selenide. His cells delivered enough current from daylight to drive a small electric motor similar to the type used in kilowatt-hour meters. Disc cells have also been developed in the Westinghouse laboratories. They use a copper-oxide disc about the size of a silver dollar. Another disc cell is produced by the Weston Company. Figure 4 shows a picture of this latter cell, called the phototronic cell. Its output is sufficient to operate a highly sensitive relay of the contact-galvanometer type, as shown in Figure 5. or to read on a microammeter. By calibrating this ammeter in light intensity, it is possible to read illumination (in foot candles) directly. For this latter purpose two cells are used together, as shown in Figure 6. The current output of the cell depends upon the light intensity and also the color of the light (its wavelength). The sensitivity maximum for this type cell is somewhat higher in the yellow part of the spectrum, the human eye having maximum sensitivity in the green-yellow part of the spectrum. In photo-cells, however, this increased sensitivity towards the longer light waves can be of advantage if they are operated from artificial light sources (as, for instance, incandescent lamps), the maximum light output of which is also moved towards the red end of the spectrum. In Figure 7 a spectral response curve of the disc cell is given. There is one more important factor which influences the current output of these cells. This is the external resistance of the circuit in which the cell operates. Like a vacuum tube or a battery, the best performance of any generator of electromotive force can be expected if its internal resistance is approximately equal to the resistance of the circuit. How this looks in the case of the photronic cell is shown in chart in Figure 8. The average sensitivity is about 1.4 microamperes per foot candle. How is a disc cell actually constructed and how does it work? In Figure 9 is shown a photograph of a device of this type, a cross-section of which is given in Figure 10. The light-sensitive element is a copper plate which has been covered with a specially prepared layer of cuprous oxide. The outer surface of this sensitive layer of minor electric conductivity is covered with a net or stripes (as clearly visible in picture No.9) which act as the second electrode. Instead of the stripes, other constructions have been used, for instance, conductive clamps attached to the outer circle and to the center of the disc. Transparent Metal Used as Second Electrode One of the most interesting constructions involved in the manufacture of these cells is the use of a continuous film of metal which covers the entire front surface exposed to the light. This conductive skin is so thin that the light is allowed to pass through the metal. On these so-called "front cells" the photo-electric effect takes place not at the main layer of the metal, but between the metal skin and the semi-conductor! The electrons liberated in this semi-conductive layer do not have to travel a considerable distance through relatively high electric resistance. It can be therefore understood that losses occurring from the high electrical resistance within the cell can be reduced considerably by construction of this type, and therefore the output of this type front cells is a better one. In the construction shown in Figures 9 and 10 this metallic cover is the positive part of the photo-cell. The second and negative terminal is the solid copper plate which the light-sensitive layer had been deposited. Various theories exist about the working of these generators of electric current which are able to transform light directly into electric energy. One hypothesis, which has been accepted today by investigators, is in principle as follows: Theory of the Disc Cell The dry cell is in its basic construction similar to the copper-oxide rectifiers. Many laws valid for the latter can be applied readily in the construction of the photo-cells. The photo current is supposed to be generated by light quanta which fall upon a semi-conductive, light-sensitive laver and generate in it free electronic oscillations. These semi-conductors have a negative coefficient of temperature in their electric resistance characteristics, The photo-electrons create an electric current by being moved through the blocking layer between the copper oxide and the main copper. This blocking layer has to be extremely thin and, at the same time, of an electrical resistance which is considerably high. It is probably this layer of a few atoms thickness that make possible not only the creation of a voltage difference (the photo-electric effect), but also the detector effect, the rectifier effect as used in the Grondahl cells6 and the reactions between minerals and metallic contacts upon them.7,8 as, for instance, argentite (Ag2S) and metal. This type of cell is dependent in its action on changes in temperature. If subjected to overheating, the sensitivity of the cell goes down. For precision measurements the temperature should be kept constant. In Figure 11 the frequency response characteristic of a cell of the type shown in Figure 9 is illustrated. We have, for instance, at 2000 cycles per second, an intensity of the output of the numeric value of 10. At about 9000 cycles this is reduced to about 50% of the amount at 2000 cycles. Will we ever get from photo-electric cells of these types a part of the electric power we need in our civilization of today? It is stated that certain laboratory types of cells are now able to deliver as much as one watt energy per square yard, in direct sunlight. Imagine desert areas, now a nuisance and a danger, covered with photo-electric power plants! Solar electric generators turning vast areas into sources of energy - without rotating machines and without considerable cost of maintenance... This is a question of further development - a development which sprouts from the needs of radio to obtain better photo-cells. References 1 Hallwachs, Ann.d.Phys., 33,301, 1888. 2 Stoletow, Journal de Physique, 9,486, 1890. 3 Elster and Geitel, Ann.d.Phys. 38,497 and by the same authors, 41,161, 1890. An exact description for the manufacturing of this type of photo-cell is given in Elster and Geitel, Phys. Zs. 1911, 609 and 1913, 714. 5 Garrison, Journ. Phys. Chem. 28,334, 1924. 6 L. O. Grondahl, USA Pat 1640335 v.T. 1.25. 7 H. H. heldnn and P. H. Geiger, Proc. Nat. Acad. Amer. 8, 161, 1922. 8 P. H. Geiger, Brit. Pat. 277610, 1927.
Posted December 23, 2022 |
||