Fuel Cells
|
|
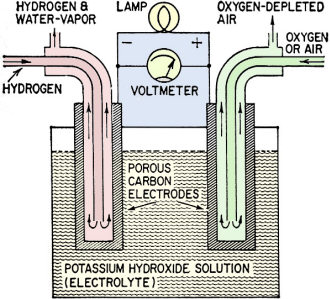
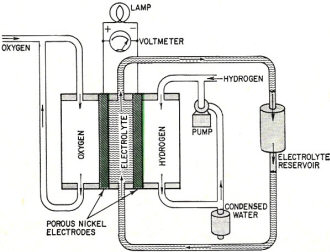
As mentioned in this 1961 Radio-Electronics magazine article, fuel cells have been around for a long time. Francis T. Bacon (not the 17th century philosopher Francis Bacon) built his first fuel cell sometime in the 1940s. He designed the fuel cells for the Apollo 11 and other spacecraft. President John Kennedy's famous moon speech was delivered a month after this article, and predated the moon landing by more than eight years. Most critical technology experiences an evolutionary period spanning decades from inception to practical application. That time is being reduced with the advent of computers and larger numbers of people working on the problems. Just as fuel cells have become ubiquitous in electrical power generation processes, so, too, will other technologies that were once considered to be pie-in-the-sky bits of science fiction - like nuclear fusion reactors (aka tokamaks). Researchers are now very near the breakeven point necessary to enable self-sustaining, controlled reactions. Fuel Cells - Tomorrow's Electric Generators? By Leonard G. Austin A fuel cell operates on the same electrochemical principle as the ordinary flashlight battery. However, a fuel is fed into the cell continually so it never runs down as long as fuel and air are available. Unlike lead-acid or nickel-iron-alkaline batteries, fuel cells do not have to be recharged electrically - "recharging" simply consists of refilling the fuel tank. Three major fields are open to fuel cells. They could be used as non-expendable cells providing cheaper and more reliable electricity than conventional batteries. Used to power electric motors they could take the place of gasoline and diesel engines for road and rail transport. Finally, large fuel-cell installations might generate electric power with greater fuel efficiency and lower cost than central power plants using boiler-steam turbine-generator systems, Fuel cells can generate small or large amounts of electricity at efficiencies up to 80%. In the past 5 years there has been an explosion of research on the cells. All major companies in the automobile, aircraft, heavy chemical, battery, electrical and oil industries are in keen competition. The most highly developed fuel cells at present use hydrogen as fuel, oxygen as oxidizer and concentrated potassium hydroxide as the electrolyte. This type of cell will be used to explain the principle of operation of fuel cells. Fig. 1 shows a simple cell of this type. The electrical energy we take from the cell as current and voltage is supplied by the chemical energy of the combination of hydrogen and oxygen to form water. Normally, hydrogen and oxygen will not react until they are heated to over 500° C. The trick in combining hydrogen and oxygen at low temperature to give electrical energy is to make the reaction proceed in a series of catalyzed steps, in one of which an electron is transferred across a circuit. (The catalysis involves a surface which holds reactants and allows them to react at much lower temperatures.) Fig. 1 - Typical hydrogen-oxygen fuel cell. Fig. 2 - What happens in a fuel cell when the circuit is closed. Fig. 3 - Open circuit stops reaction.
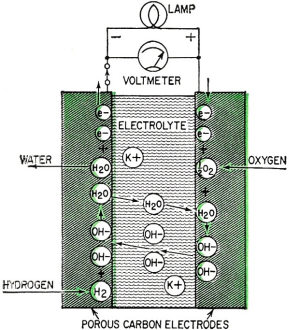
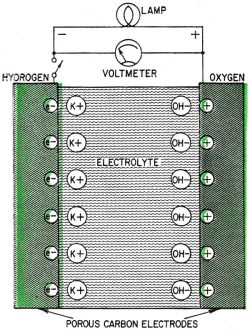
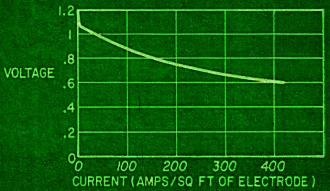
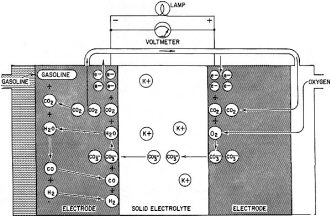
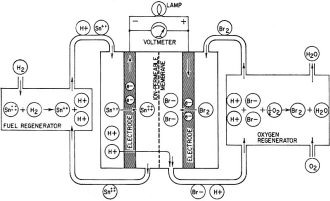
Fig. 4 - Current-voltage curve of a hydrogen-oxygen fuel cell. Fig. 5 - Francis T. Bacon developed this hydrogen-oxygen cell. Fig. 6 - High-temperature fuel cell uses "solid" electrolyte of potassium carbonate and operates at temperatures above 500°C. Fig. 7 - Redox type fuel cell. Fig. 2 shows the series of steps in a fuel cell. It has two porous electrodes separated by a potassium hydroxide electrolyte. On the negative side of the cell, hydrogen gas diffuses through the electrode, and hydrogen molecules (H2), assisted by a catalyst embedded in the electrode surface, are adsorbed on the surface in the form of hydrogen atoms (H). The atoms react with hydroxyl ions (OH-) in the electrolyte to form water, giving up electrons to the electrode in the process. The water goes into the electrolyte. This reaction is also aided by the catalyst. The flow of these electrons through the external circuit to the positive electrode is the electric output of the cell and supports the oxygen half of the reaction. On the positive side of the cell oxygen (O2) diffuses through the electrode and is adsorbed on the electrode surface. In an indirect reaction, the adsorbed oxygen, plus the incoming electrons and water in the electrolyte, form hydroxyl ions. Here again a catalyst helps the reaction along. The hydroxyl ions complete the circle by migrating through the electrolyte to the hydrogen electrode. If the external circuit is open, the hydrogen electrode accumulates a surface layer of negative charges that attracts a layer of positively charged sodium or potassium ions in the electrolyte. An equivalent process at the oxygen electrode similarly balances its accumulated positive charge (Fig. 3). These electrical "double layers" prevent further reaction between the gases and the electrolyte. These layers also provide the potential that forces the electrons through the external circuit when an external connection is made. Theoretically, a fuel cell can convert the available chemical energy of the fuel to electrical energy at almost 100% efficiency. In practice, energy has to be used to overcome the activation energy barriers of the reactions in the cell, overcome the ohmic resistance of the electrolyte and supply the transport energy of feeding the gases through the porous electrode. High current drain makes these losses greater and the cell voltage decreases from the open circuit value (Fig. 4). Efficiencies of 60% to 80% at working currents can be obtained. If we compare this efficiency level with 25-30% for the normal gasoline engine and 35-40% for central electricity power generation, we see why fuel cells are exciting so much interest. Union Carbide has developed a hydrogen-oxygen cell which operates at about 60° C. The electrodes consist of porous carbon impregnated with catalysts: fine particles of platinum or palladium in the hydrogen electrode and cobalt oxide, platinum or silver in the oxygen electrode. To prevent flooding the pores by the electrolyte - which would cut down the active surface - the electrodes are waterproofed with paraffin wax. The wax prevents the water from creeping into the pores. To bring the electrodes closer together and thus speed ion transport, they are usually arranged as concentric tubes or adjacent plates. These cells are being used by the Army as portable, quiet power generators. General Electric has developed a low-temperature hydrogen-oxygen cell which uses an ion-exchange membrane instead of a liquid electrolyte. The membrane transports a hydrogen ion (H+) to the oxygen electrode where it reacts to form water. The electrodes are thin sheets of porous platinum-palladium. This cell is suitable only for small current outputs because of the low ionic conductivity of the membrane; however, it is thin and about 60 cells stacked in series are but 1 foot long. If the electrochemical reactions are speeded up by running the cells at higher temperatures, hydrogen-oxygen cells are capable of much greater electrical outputs per unit volume. To prevent electrolyte boiling at high temperatures, the whole cell must be pressurized. A cell of this type, developed by Francis T. Bacon of Cambridge University (England), operates at about 250° C with gas pressures that run as high as 800 pounds per square inch (Fig. 5). The porous nickel electrodes are about 1/16 inch thick, usually in the form of disks or plates. The reaction area is a thin, porous surface layer on the electrode. The electrolyte is a solution of potassium hydroxide and could enter these pores but for the pressure differences within the electrode which keep the electrolyte from flooding the larger pores in the body of the electrode. This fuel cell produces six times as much power per cubic foot as the low-temperature hydrogen-oxygen cells. High-Temperature Cells Although hydrogen-oxygen cells have been well developed, they have two big disadvantages. Hydrogen is costly and bulky. Hydrogen stored at 150 atmospheres pressure contains less than one-hundredth the energy of the same volume of gasoline at normal conditions. Fuel cells must "burn" cheap fuels (natural gas, vaporized gasoline) to produce economical power on a large scale. Extracting energy from such fuels at present needs operating temperatures above 500°C. Since water-base electrolytes boil away at these temperatures, the electrolyte usually consists of some molten salt, sodium or potassium carbonate, mixed with lithium carbonate to lower the melting point for example. In the most efficient high-temperature cells, the electrolyte is held in a matrix of porous refractory material. The electrodes, made of a variety of metals or metallic oxides, are tightly pressed against the "solid" electrolyte. In these cells the fuel is probably "cracked" to hydrogen and carbon monoxide by reaction with steam and carbon dioxide, which the fuel cell produces as byproducts. This cracking may be done outside or inside the cell. In the current-generating reaction, the hydrogen and carbon monoxide diffuse into the cell at the negative electrode, react with carbonate ions in the electrolyte and form carbon dioxide and water while giving up electrons to the electrode. At the positive electrode, oxygen or air takes up the electrons flowing in from the external circuit, reacts with the carbon dioxide and produces the carbonate ions. Migration of carbonate ions through the electrolyte from the positive to the negative electrode completes the circuit (Fig. 6). High-temperature fuel cells, intensively investigated only since World War II, still perform poorly. The best of them produce no more than half the yield of the low-temperature hydrogen-oxygen cell and a twelfth the yield of the Bacon cell. However, the progress already made in hydrogen-oxygen cells suggests that further research can greatly improve the performance of high-temperature cells. Another cell which might be able to use cheap fuels is the "redox" cell (named for reduction and oxidation). In this cell the fuel and oxygen are made to react with other substances in "regenerators" outside the cell to produce chemical intermediates, which in turn generate current in the cell. The overall reaction is the same as that of combustion, however, because the intermediates are regenerated. A typical cell of this type was developed in England under the leadership of Sir Eric Rideal. It uses tin salts and bromine as intermediates (Fig. 7). The fuel reduces (adds electrons to) tin ions, which then give up the added electrons to the negative electrode and return to react with more fuel. The oxygen similarly oxidizes (takes electrons from) bromide ions, converting them to bromine, which then takes up electrons from the positive electrode and returns as bromide ions for regeneration. A similar cell, using titanium salts instead of tin, is under development by the General Electric Co. The stumbling block with this type of cell is that most fuels cannot react quickly enough in the regenerator to keep up the current flow. Investigations of catalysts to speed up the reaction are being made by G-E and by the Pennsylvania State University. Special-Purpose Cells Several other types of cells and many fuels are being investigated. Hydrazine (N2H2) and ammonia (NH3) are being used as fuels in hydrogen-oxygen type cells. They act as convenient hydrogen carriers; a catalyst in the electrode splits them into hydrogen and nitrogen. Methyl alcohol (wood spirit) has been used in similar cells. Although alcohol is not cheap enough to use for large-scale electricity production, it is certainly cheap and convenient enough to replace expensive nonrechargeable dry cells. Allis-Chalmers Co. has developed a cell which runs at 60°C on a fuel mixture of propane and hydrogen. It uses potassium hydroxide electrolyte and activated nickel electrodes. A 20-horsepower tractor has been built using 1,000 of these cells for the power unit. The Electric Storage Battery Co. is developing a cell originated by E. Yeager at Western Reserve University. The fuel is sodium, dissolved in mercury, which reacts with oxygen and water to form sodium hydroxide. It will not produce cheap electricity because of the high cost of sodium, but it may have important defense applications such as powering a quiet submarine. It does not require a vivid imagination to picture the possible applications of fuel cells. Applications range from electric cigarette lighters fueled with methyl alcohol, to heavy power production for electrical furnaces used by such industrial giants as the aluminum, glass and steel industries. A fuel cell "burning" gasoline or light hydrocarbons could be the power unit of an automobile if it had a satisfactory power to volume ratio, long life and rapid startup. An electric automobile requires no transmission: it could have electrical braking, four-wheel drive and differential wheel speeds for safe cornering. Troubleshooting on such an automobile would be done mainly with a test meter. There is no doubt that the use of fuel cells will need specially designed electrical and electronic control and testing gear plus engineers capable of using them. However, I should like to emphasize that I have not discussed the many disadvantages and problems remaining to be overcome in the development of fuel cells. It is possible that many of the potentialities of fuel cells will never be reached, but fuel cells in some form for some applications are on the way. Further Reading G. J. Young, Fuel Cells, Reinhold Publishing Corp., 1960. B. R. Stein, Status Report on Fuel Cells. ARO Report No. 1. Office of the Chief of Research and Development, Department of Army, Washington 25, D. C. ($1.25)
Posted May 15, 2023 |
|