The Carnot Cycle |
||||||||||||||
|
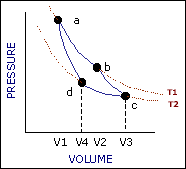
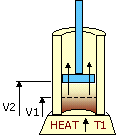
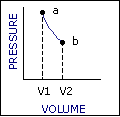
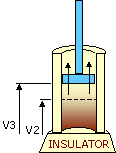
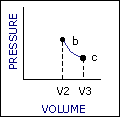
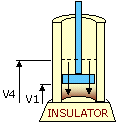
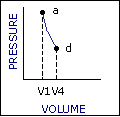
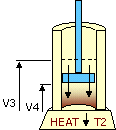
An ideal cycle would be performed by a perfectly efficient heat engine—that is, all the heat would be converted to
mechanical work. A 19th-century French scientist named Nicolas Carnot conceived a thermodynamic cycle that is the
basic cycle of all heat engines. He showed that such an ideal engine cannot exist. Any heat engine must expend
some fraction of its heat input as exhaust. The second law of thermodynamics places an upper limit on the
efficiency of engines; that upper limit is less than 100 percent. The limiting case is now known as a Carnot
cycle.
The Carnot Cycle
|
||||||||||||||