|
Electricity - Basic Navy Training Courses NAVPERS 10622 |
|
Here is the "Electricity - Basic Navy Training Courses" (NAVPERS 10622) in its entirety. It should provide one of the Internet's best resources for people seeking a basic electricity course - complete with examples worked out. See copyright. See Table of Contents. • U.S. Government Printing Office; 1945 - 618779
Chapter 1 A black night-no moon and a few stars here and there. Gun crews are alerted
by enemy aircraft. Guns are pointed, trained, and fired. How are those guns pointed
and trained? By hand? No! Sighting is impossible. The guns are entirely on Director
Control. All of this work-locating the target, training and pointing, and even firing-is
done by ELECTRICITY.
Let's see what all this means. If you crushed a common brick, you would get a pile of small grains of sand and clay. These grains are matter just as the whole brick was matter. According to the scientists, you have not made any changes in this matter. The sand and clay could be remolded and baked. You would again have a brick. Now, if you COULD break down one grain of sand into its smallest parts (without destroying the sand), you would have billions upon billions of MOLECULES. Molecules are almost unbelievably small. It would take 300,000,000 molecules laid end to end 'to make a line one inch long. The molecule is the smallest particle of any piece of matter that can exist and STILL BE the SAME KIND OF MATTER.
As small as the molecule is, it is not the smallest particle of matter. Every
molecule can be broken down into two or more smaller particles called ATOMS. But
you would NO LONGER have the same kind of matter. Two DIFFERENT substances are obtained
from the break-up of the sand molecule a gas (oxygen) and a solid (silica).
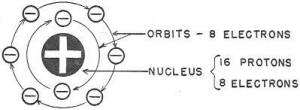
These are ELEMENTs - the building materials of matter. The smallest particle of
each of the different elements (and there are only 92 different elements) is an
ATOM. Thus you obtained ATOMS of oxygen and ATOMS of silica from a MOLECULE of sand.
You can reverse the "tearing down" process to one of "building up "-
two or more atoms are combined to form a molecule. Molecules of steel, copper, water,
rubber, paint, oil, -in fact, ALL SUBSTANCES - are simply combinations of two or
more atoms.
CONSTRUCTION OF the MOLECULE It is easier to understand how a ship. is constructed if you work in a shipyard. You see the keel laid, frames set in place, stanchions erected, and the decks laid. Then, during outfitting, you see the wiring installed, tackle and gear set in place, and the guns swung aboard. In short, you see the ship constructed from its smallest parts. It would probably be easier to understand how a piece of matter is constructed if you could see it built. But, unfortunately, there is no microscope powerful enough to permit you to see electrons, protons, atoms, or even molecules of matter.
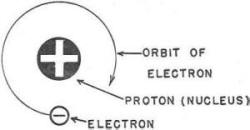
However, suppose you do a little imaginary enlarging-you have increased the sizes of electrons and protons until each electron is represented by a small white marble, and each proton is represented by a somewhat larger black marble.
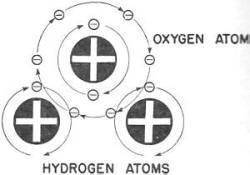
Figure 1. - The hydrogen atom. Now - you are going to construct enough water to fill a drinking glass. You will need billions of small white marbles (electrons) and exactly the same number of larger black marbles (protons). Anything else for ingredients? No! All matter - water, steel, brick, gunpowder, air, and even YOU - is made only of ELECTRONS and PROTONS. Water consists of two atoms of the element hydrogen and one atom of the element oxygen. You'd build the hydrogen atom first as it contains only one proton and one electron. You anchor one proton (black marble) to form the center, or stationary nucleus, of your hydrogen atom. Then you spin an electron (white marble) around this nucleus. You have one atom of hydrogen! It would look like figure 1.
Second, you must build another hydrogen atom, because each molecule of water
contains two hydrogen atoms.
Figure 2. - The oxygen atom. notice that the rapidly rotating electrons of both the oxygen a tom and the two hydrogen a toms cross each other's orbits or paths. This locks the atoms together. The stationary nuclei of the atoms are held in place by the attraction of their electrons. (You will learn more about this attraction later.)
Figure 3. - A molecule of water. Perhaps you remember the German dirigible Hindenberg. This was a hydrogen-filled ship. Some of 'the hydrogen leaked from its tanks and mixed with the oxygen of the air. A spark set off this mixture as the ship landed at Lakehurst. The resulting explosion formed quite a bit of water and, incidentally, killed most of the crew.
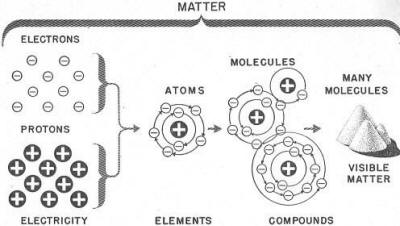
You now have a pretty good idea of the structure of water. Water, however, is only one kind of matter. Other kinds, steel, wood, air, cloth, and food, are built up the same way. Protons and electrons combine to form atoms of the 92 elements. The atoms combine to form molecules. And molecules-pack together until they form a bit of matter large enough to see. Remember, if you break any-thing down _to the smallest possible part, you will have positive bits of electricity - the PROTONS - and negative bits of electricity - the ELECTRONS. A little study of the chart in figure 4 will help
you to remember these units of matter. Figure 4. - Electricity and matter. Chapter 1 Quiz
|